Burning Rock's Technology
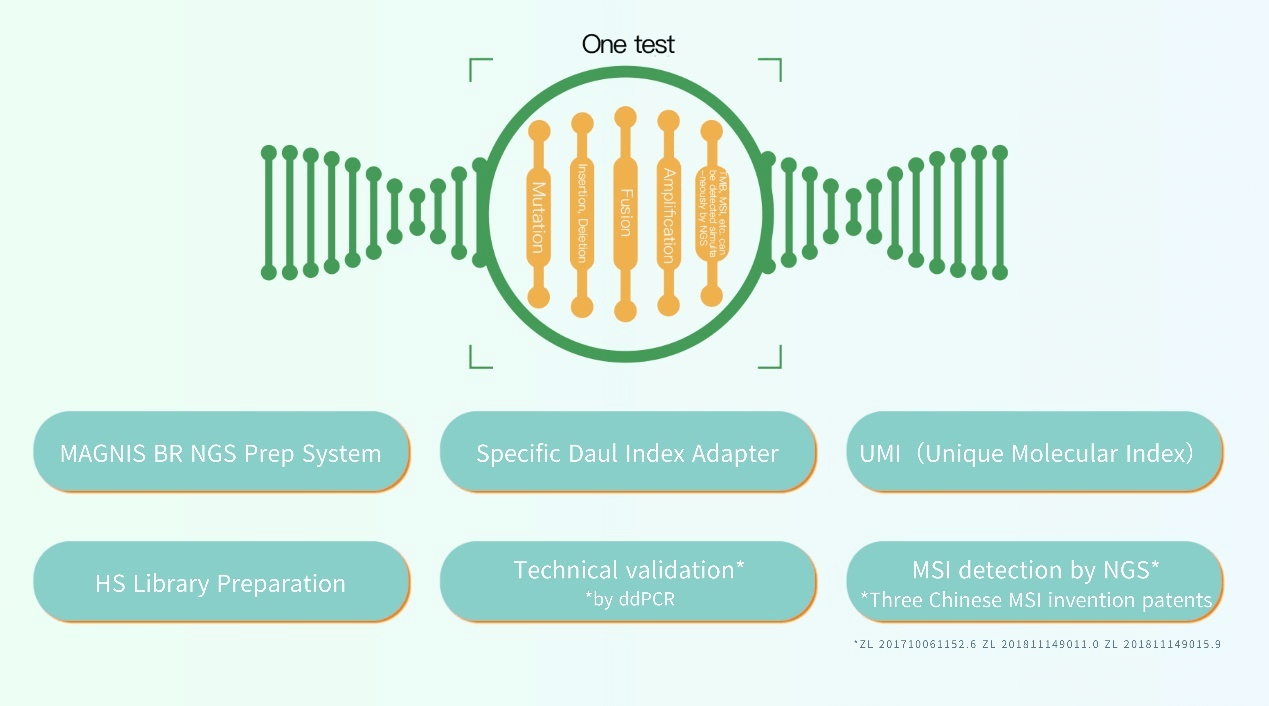
Burning Rock has independently developed a number of industry-leading technologies. Our workflow is adapted to Illumina's platforms such as NextSeq 2000 and NextSeq 550Dx. The library construction and target enrichment steps feature our proprietary chemistry and we have the unique capability of performing both hybridization-based and amplification-based protocols.
Our gene panels have been validated through the independent assessment to have industry-leading performance. We were chosen as a vendor to participate in the SEQC2 (sequencing quality control phase 2) study, led by an FDA (the U.S. Food and Drug Administration)-led community wide consortium (the MAQC consortium) to assess the performance of oncology panels. This study evaluated the performance of liquid biopsy panel and tissue panel respectively. The panels were tested using a synthetic blend of gDNA from cancer cell lines (Agilent UHRR) diluted with gDNA from normal cell lines to mimic the low frequency of tumor DNA in real clinical samples. The results were then compared against the established “truth set” of known positives and known negatives to measure assay performance.
In evaluating the performance of pan-cancer tissue panels, among the 5 vendors that participated in the study including Roche, IDT, Illumina, BR, and Thermo, we are proud our OncoCompass™ Target presented with high sensitivity, specificity and reproducibility, ranking #1 in comprehensive performance.



In evaluating the performance of pan-cancer tissue panels, among the 8 vendors that participated in the study including Agilent, IDT, Illumina, Qiagen, Roche, Thermo, BR, and Genentech, we are proud to consistently place among the leading performers at all dilution ratios. Our assay showed consistent sensitivity over 94%. We ranked #2 in sensitivity and limit of detection.