- Professional Cross-functional Team
- Operational Excellence
- Transparent Quality Control and Reaction Systems on Abnormal
- International Logistics and Chain of Custody
- Customized Documentation Services for Clinical Trails
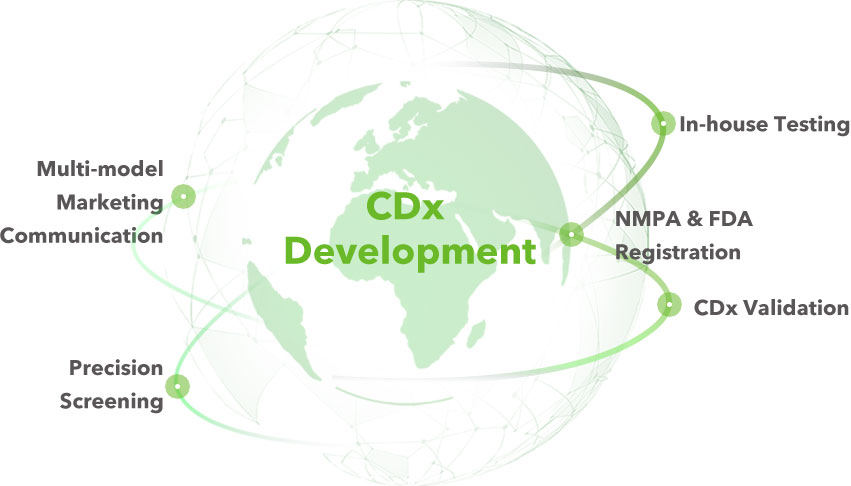
- Raw material & preparation
- Production procedure evaluation
- Technical requirements proposal
- Product stability evaluation
- Product validation analyses
- Cut-off value & coverage assesment
- Standardized production procedure