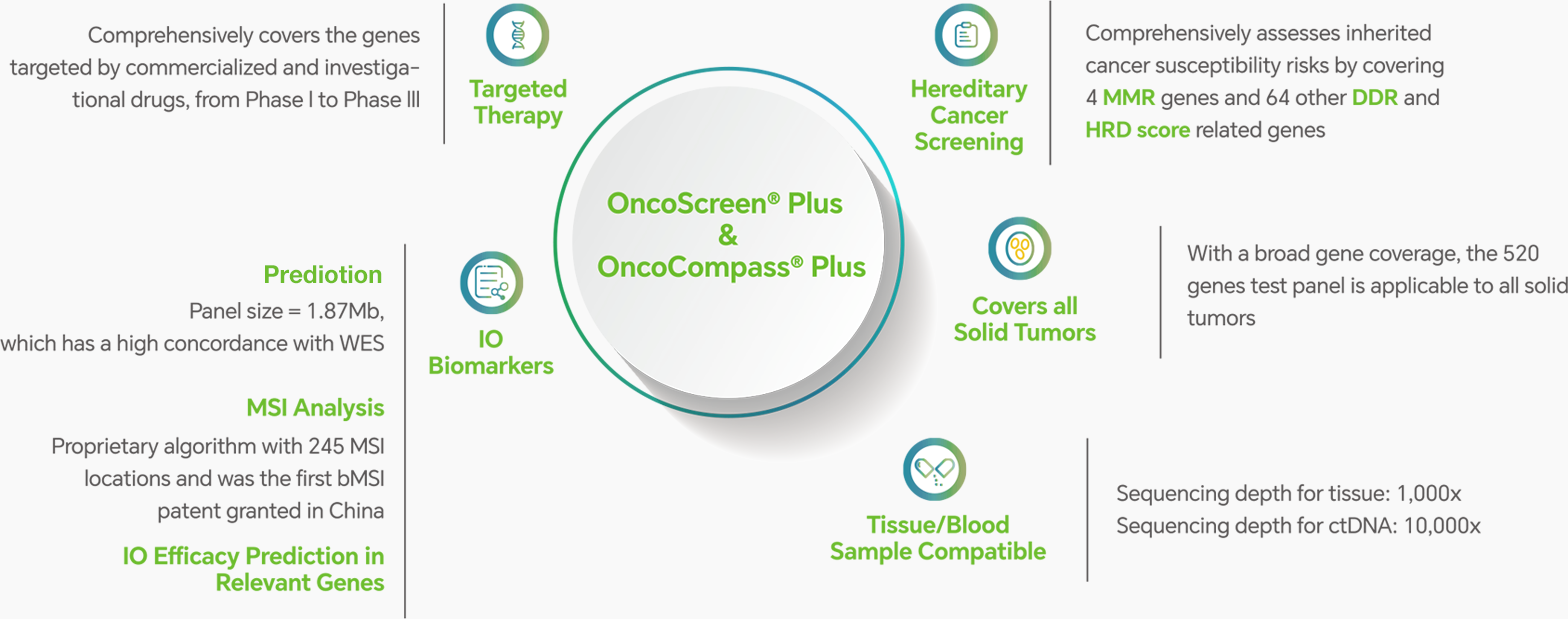

● Note: Different mutation types demonstrated a consistent >97% sensitvity and >95% specificity in clinical sample validation tests.

Taking part in more than 110 clinical trial assays including CS 1001 and Tislelizumab (4 CDx and 9 Pilot Stidies)

Activating mutations in PIK3CA and AKTI and inactivating alterations in PTEN genes were determined centrally (after randomization) by means of next-generation sequencing with the use of the Foundation One CDx assay (Foundation Medicine) in all countries except China (OncoScreen® Plus,Burning Rock Biotech).
OncoScreen® Plus has been included in 165 SCI publications,the accumulated impact factor has reached 1097,more than 20 papers have IF>10,including one in The New England Journal of Medicine.(IF=158)