● Efficient and Accurate Library Construction
Utilizing Single-Tube-Hybridization, Anti-Contamination-Adapter, and Dual-End-Barcode techniques, the kit ensures rapid and simplified operation, minimizing the risk of contamination while maximizing conversion efficiency.
● Intelligent and Efficient Data Analysis
OncoMaster, a comprehensive data analysis system, integrates a robust knowledge base and advanced patented algorithms along with high-performance analysis processes, enabling rapid and efficient data interpretation.
● Small in Size, Big on Performance
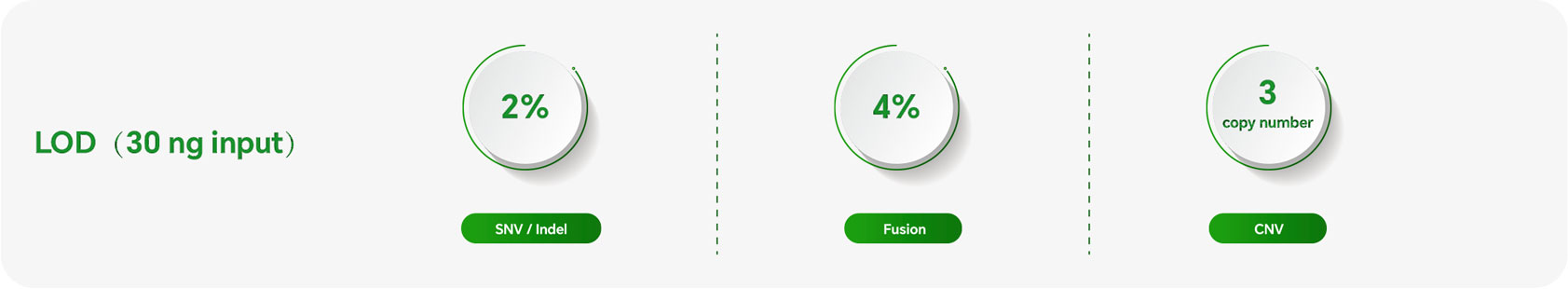
Despite its compact size of 53kb, the panel covers the vast majority of variants in tierⅠ and tierⅡ associated with lung cancer, colorectal cancer, and gastrointestinal stromal tumors.
● Excellent User Experience
The kit’s operation is straightforward and user-friendly, ensuring a seamless experience for all users. Equipped with the fully automated library preparation system, Nexus BR, NGS detection becomes even more convenient.
 Non-small cell lung cancer (NSCLC):
Non-small cell lung cancer (NSCLC):

 Colorectal cancer (CRC):
Colorectal cancer (CRC):
 Gastrointestinal stromal tumor (GIST):
Gastrointestinal stromal tumor (GIST):